Harnessing the sun to create hydrogen fuel
Story by Cheryl Pierce
Clean hydrogen as a fuel source is currently used in industry, but it is produced by water electrolysis without directly using sunlight. Researchers at Purdue University are attempting to simplify the process by producing hydrogen fuel from water using sunlight. Up until now, no efficient device has been devised that would allow this process to occur.
Being able to create hydrogen using sunlight would revolutionize the hydrogen industry. With this process, storage of intermittent sun energy would be much more efficient. Collected hydrogen could be used at any time when energy is needed or supplied for use in the chemical industry.
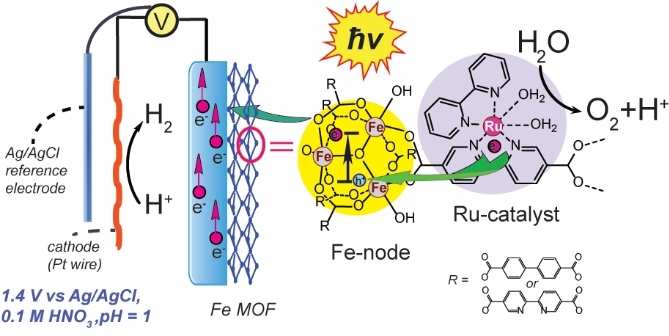
The team, led by Dr. Yulia Pushkar, Professor of Physics and Astronomy from the Purdue Department of Physics and Astronomy, published plans for their device in ChemSusChem, a journal of European Chemical Society, “Photoexcitation of Fe3O Nodes in MOF Drives Water Oxidation at pH=1 When Ru Catalyst Is Present.”
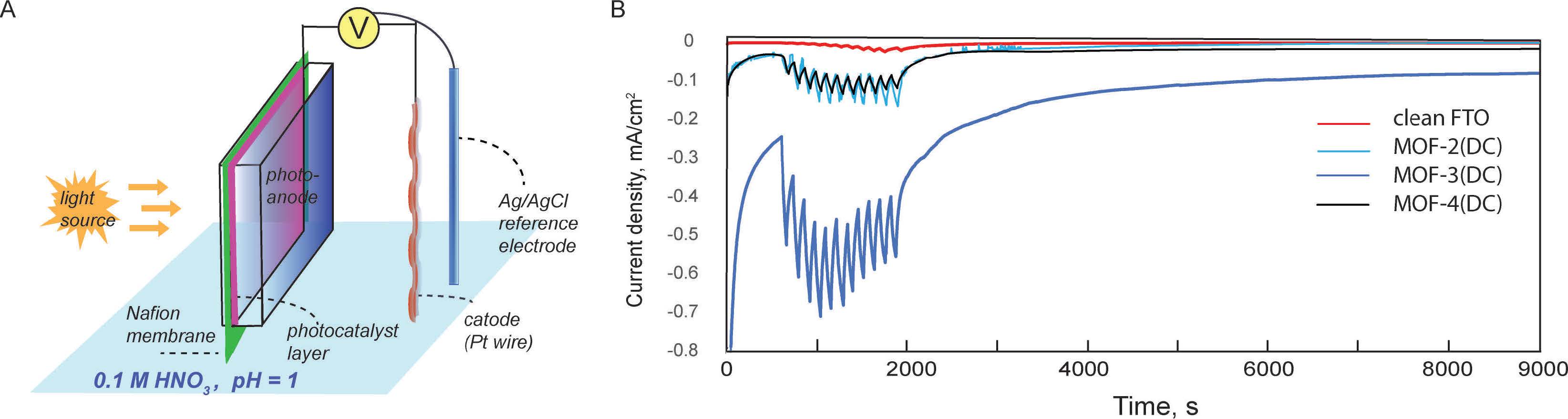
Generally, experts thought that a device that could be capable of creating hydrogen on industrial scale would operate at pH=1 similar to most efficient proton exchange membrane (PEM) electrolizers. However, the device conceptualized at Purdue uses light driven water splitting at pH=1. This is possible due to suitable materials in the device which would be both active with visible light and stable at pH=1.
The research team consisted mostly of researchers from Pushkar’s lab. Dr. Roman Ezhov, postdoctoral research associate, led the study while Dr. Alireza Ravari, former graduate student, helped with laser-based measurements. Dr. Sergei Savikhin, Professor of Physics and Astronomy from the Purdue Department of Physics and Astronomy, provided access to femtosecond laser system. Alexander Loomis, former undergraduate student at Purdue, also helped conduct research. Researchers outside of Purdue included Argonne Scientist, Debora M. Meira, who assisted with remote data collection at Advanced Photon Source and Dr. Mark Palenik, Purdue Physics and Astronomy alumnus, who is a long term theoretical collaborator on the project.
“We learn from natural photosynthesis which converts the sunlight into chemical fuels,” says Pushkar. “Here we have Metal organic framework (MOF) material which works like Lego bricks. We put together chlorophyll analog = Fe3O node and photosystem II active center analog = molecular Ru catalysts. When we shine light, they work together like in the photosynthetic protein and split water into O2, protons and electrons. But the MOF system is much more stable than a protein as it is a 3-dimentional porous material. Electrons ripped off water molecules are passed through the wire and combine with protons on cathode to make hydrogen.”
According to Pushkar, one industry that might be drastically changed by a usable device that harnesses the power of the sun to create hydrogen is the fertilizer industry. She says the production of fertilizers currently use "dirty and unsustainable" hydrogen generated from breaking methane (natural gas, CH4, note also price fluctuations).
The next giant leap in hydrogen creation is an ongoing work in progress. Pushkar’s lab continues to work on this device in the experimental phase. Purdue researchers have been working on this issue since 2008 and this is the first viable device created.
“All prior systems we researched were too far removed from any hope of practical applications,” says Pushkar. “Purdue allowed the team to start a long-time collaboration with former PhD graduate Dr. Palenik and to work with the Savikhin lab which allows for additional spectroscopic characterization.”
Funding for this research is provided by the National Science Foundation and is on-going (CHE-2155060).