Cracking nature’s code: New insights into oxygen formation could revolutionize artificial photosynthesis
2025-02-19
 Running the time-resolved X-ray emission experiment at the BioCARS beamline at Advanced Photon Source after long spectrometer development and experiment preparation. Green laser light is visible as it advances the Photosystem II protein through the Kok cycle. Photo provided by Yulia Pushkar.
Running the time-resolved X-ray emission experiment at the BioCARS beamline at Advanced Photon Source after long spectrometer development and experiment preparation. Green laser light is visible as it advances the Photosystem II protein through the Kok cycle. Photo provided by Yulia Pushkar.
Current and former Purdue University researchers have made strides in understanding a critical step in photosynthesis. They have zoomed in on the moment oxygen is formed, potentially avoiding harmful byproducts and paving the way for designing artificial systems to replicate photosynthesis.
It's well known that plants use sunlight to create energy, release oxygen, and absorb carbon dioxide. The oxygen comes from a specialized photosystem II protein, which contains an oxygen-evolving complex (OEC) involving light that splits water into its basic parts: protons, electrons, and oxygen. While we know that the OEC contains a Mn4Ca cluster that cycles through five so-called S-states (S0–S4) to make this happen, the exact step where oxygen atoms bond together remains a mystery scientists are still working to understand. Researchers can get an understanding of the formation of the O-O bond through time-resolved X-ray diffraction, infrared spectroscopy, computation DFT, and QM/MM techniques.
Yulia Pushkar, a professor at the Purdue Physics and Astronomy Department, along with former graduate students Scott Jensen, Brendan T. Sullivan, former postdoc Daniel A. Hartzler, and former physics undergraduate student Allison Page are all involved with this research, which has been published in Chem. Outside of Purdue University, Irina Kosheleva and Robert W. Henning are beamline scientists at the Advanced Photon Source, Argonne National Laboratory in Lemont, Illinois. They developed experiment implementation at the specialized beamline (BioCARS).
“My research is interdisciplinary and lies at the interface of physics, chemistry, and biology,” says Pushkar. “It has a niche where modern powerful experimental (such as lasers and modern X-ray sources) tools are used to understand how biological systems work from brains to proteins to molecules and non-biological materials (catalysts, battery materials).”
Pushkar and her team expanded the use of a time-resolved X-ray emission spectroscopy technique, which can detect changes in the Mn4Ca cluster at the heart of the process. This method allowed them to track the reactions inside the protein complex with incredible speed, measuring events with microsecond resolution. The experiments were conducted at a high flux beamline at the Advanced Photon Source, Argonne National Laboratory.
“In the final catalytic step responsible for O2 formation (S3-to-S0 transition), the measured spectroscopic changes are consistent with the early reduction of the Mn centers (in comparison to the S3 state) ~50-200 ms in H2O and ~50-500 ms in D2O, ahead of the reduction of the redox-active TyrZ·+,” explains Pushkar. “These results indicate the multi-step nature of the oxygen-oxygen (O-O) bond formation and O2 release by the OEC, where an O-O bond is likely formed prior to the final electron transfer step.”
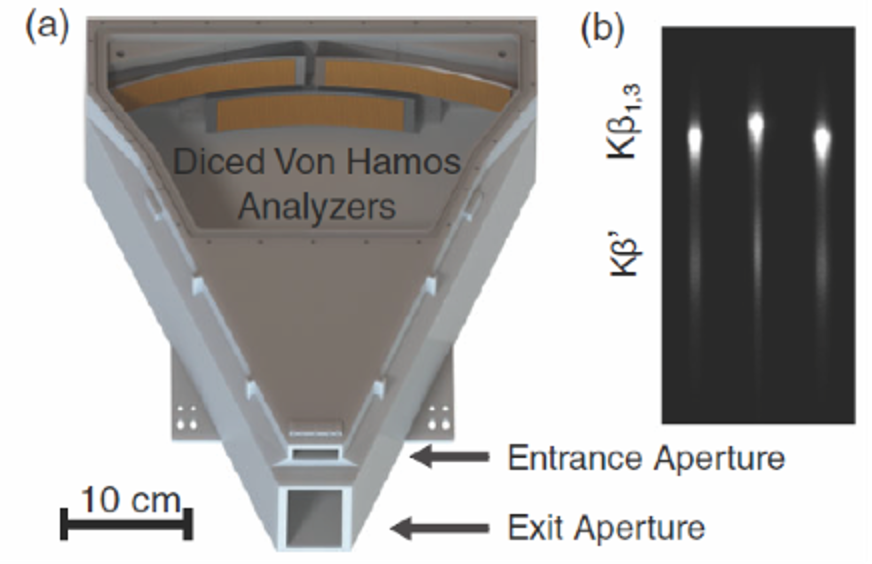
(a) The 3D‐printed spectrometer with three von Hamos analyzers inside. Analyzers are shown in orange for visibility. (b) The Pilatus 100 k image of the emission spectra from MnO showing the reflection of each of the three analyzers. S. Jensen, X-ray spectrometry, 2019.
This could be an important evolutionary adaptation that helps prevent the release of harmful byproducts or peroxo species. Using a special form of water (D2O), the team extended the time up to ~500 ms to study an early, unstable state of possible peroxo nature, opening it to further spectroscopic and structural analysis. These insights could eventually help create better catalysts for artificial photosynthesis, mimicking nature's process for producing energy.
At the end of the day, “The goal is to follow individual atoms and electrons in space and time when the catalytic process of light-induced O2 formation is taking place inside Photosystem II protein,” says Pushkar.
This discovery is key to advancing artificial photosynthesis. A new understanding of how Photosystem II works will directly benefit ongoing research in this field. In artificial photosynthesis, the sunlight's energy is used to produce storable fuels like hydrogen by splitting water into hydrogen and oxygen using a special device.
“I have worked on the analysis of photosynthetic proteins since 2000 and my research group worked on the studies of Photosystem II since 2008 when I joined Purdue as a biophysics faculty. At Purdue, we conducted both experimental and computational analyses, as exemplified by our prior publication,” explains Pushkar. “Earlier computational work was aided by the Rosen Center for Advanced Computing at Purdue. Purdue has other faculty who work in the Biophysics of Natural Photosynthesis in the College of Science and other Colleges.”
The research was funded by the National Science Foundation. This work was mostly done under an older award (CHE-2004147), but its continuation is ongoing CHE-2303743.
About the Department of Physics and Astronomy at Purdue University
Purdue’s Department of Physics and Astronomy has a rich and long history dating back to 1904. Our faculty and students are exploring nature at all length scales, from the subatomic to the macroscopic and everything in between. With an excellent and diverse community of faculty, postdocs and students who are pushing new scientific frontiers, we offer a dynamic learning environment, an inclusive research community and an engaging network of scholars.
Physics and Astronomy is one of the seven departments within the Purdue University College of Science. World-class research is performed in astrophysics, atomic and molecular optics, accelerator mass spectrometry, biophysics, condensed matter physics, quantum information science, and particle and nuclear physics. Our state-of-the-art facilities are in the Physics Building, but our researchers also engage in interdisciplinary work at Discovery Park District at Purdue, particularly the Birck Nanotechnology Center and the Bindley Bioscience Center. We also participate in global research including at the Large Hadron Collider at CERN, many national laboratories (such as Argonne National Laboratory, Brookhaven National Laboratory, Fermilab, Oak Ridge National Laboratory, the Stanford Linear Accelerator, etc.), the James Webb Space Telescope, and several observatories around the world.
Written by: David Siple, Communications Specialist, Purdue University Department of Physics and Astronomy
Contributors:
Yulia Pushkar, professor, Purdue University Department of Physics and Astronomy