Demos: 3A-05 Gas Expansion
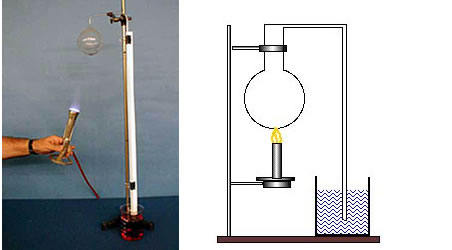
The bulb and the long stem initially contain air. The air is heated, the burner is removed and the long stem is placed in a beaker of colored water. As the air cools, water is seen to rise up the tube. The visible part of this demonstration is actually illustrating the contraction of the gas as it cools, but the principle is the same as for expansion.
Directions: The tube may be placed in the water ahead of time, in which case the heating of the air will cause bubbles to pass into the water. Turn off the burner and watch the water rise in the tube.
Suggestions for Presentation: Because air is transparent, it would be difficult to show its expansion, as we did with liquids and solids. Tell the students that we are going to infer that the gas expands when heated by noting that it contracts when cooled. This is sometimes referred to as an indirect experiment. The cooling causes the pressure to drop inside the bulb so that the atmospheric pressure pushes the liquid up into the tube.
Applications: Old “air thermometers” were based on this principle. The problem with these is that atmospheric pressure changes also affect the height of the column in the tube.
Last Updated: Nov 30, 2023 11:25 AM