Demos: 2A-12 Boyle’s Law
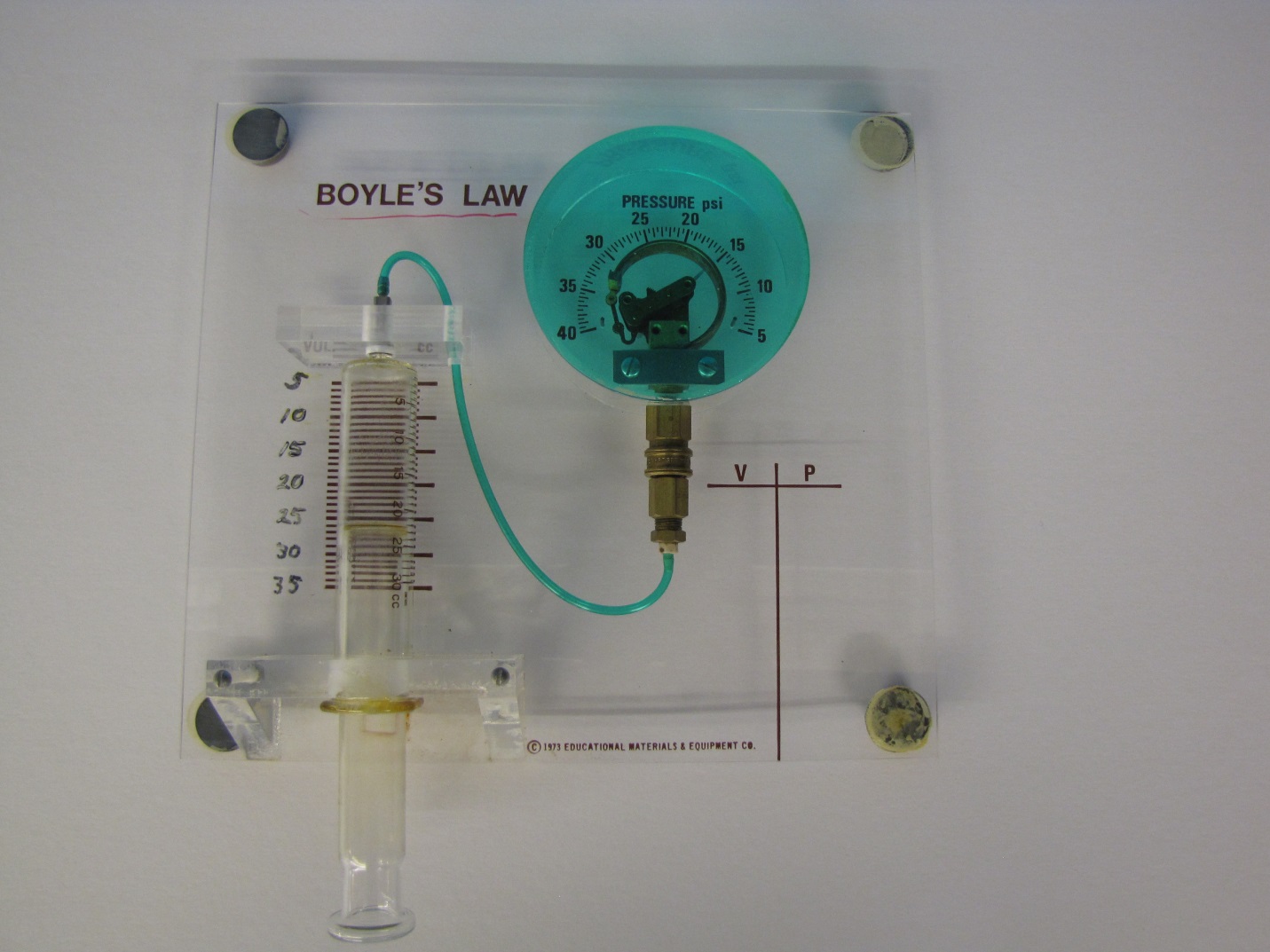
This is an accurate device for illustrating the basic relationship between pressure and volume of gases, known as Boyle’s Law. The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and the amount of gas remain unchanged in a closed system. A calibrated syringe is connected to a pressure gauge. As you change the volume in the syringe you can show the change in pressure on the gauge.