|
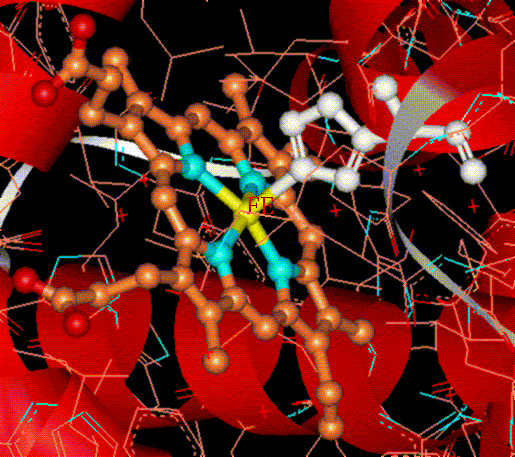
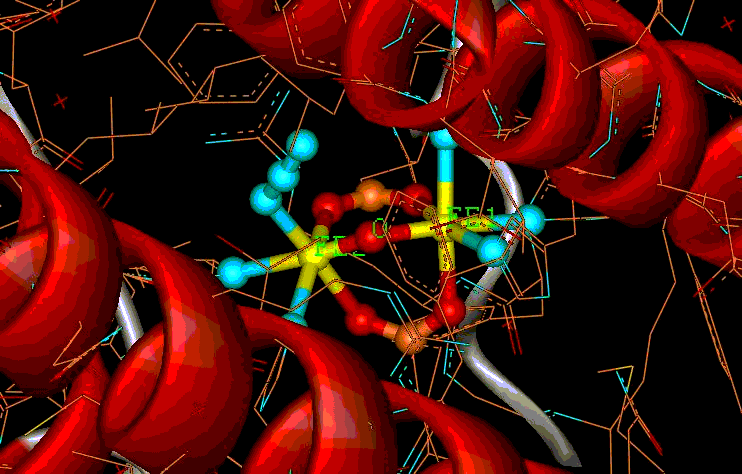
COMPUTATIONAL ELECTRONIC STRUCTURE OF METALLOPROTEINS First principle (ab initio) methods provide significant insight about the electronic structure and physical properties of structures of interest in physics, chemistry, biology and materials science. Some of these methods are useful for understanding the electronic structure of active sites in metalloproteins and for interpreting experiments that probe their ground or excited states. We apply density functional theory (DFT) and other ab-initio methods to extract physical and chemical information from calculated electronic densities or many-body wavefunctions. In addition, we apply methods of ligand field theory (LFT) and robust algorithms (e.g., genetic algorithms) to simulate complex experimental data from resonance spectroscopies (e.g., Mössbauer, EPR). We are also exploring computational approaches for the interpretation of biological X-Ray (XANES, RIXS) spectra. Hemoglobin
Hemoglobin is an oxygen transporting protein whereby O2 binds reversibly to iron-porphyrin active sites. Upon binding of O2 the iron-porphyrin complex undergoes subtle structural rearrangements with a concomitant change from the ferrous (deoxyhemoglobin) to the ferric (oxyhemoglobin) oxidation states. We are studying the electronic structure of oxyhemoglobin within the framework of density functional theory (DFT).Simulations of the binding process were carried out which show that the orientation of O2 with respect to the porphyrin plane follows a specific trend which minimizes the overall electronic energy.
Diiron-Oxo Proteins
Oxo- and hydroxo-bridged
diiron centers are ubiquitous in biology. The importance of these structural
motifs is illustrated by the name given to an entire class of metalloproteins,
namely the "diiron-oxo proteins". The family of hemerythrins (Hr),
in particular, are oxygen-transporting proteins that can be considered the
best-characterized of the class. Combined crystallographic and spectroscopic
data show that oxy-Hr and other derivatives, such as azidomet-Hr, contain
strongly antiferromagnetically coupled oxo-bis(carboxylato)-bridged Fe3+-Fe3+ centers.
By contrast, deoxy-Hr contains a weakly antiferromagnetically coupled hydroxo-bis(carboxylato)-bridged
Fe2+-Fe2+ center. Thus, in spite of their structural
similarity, there are meaningful differences in electronic structure among
the various forms of hemerythrin. To gain insight about the specific electronic
structures and magnetic properties of each diiron-oxo protein
we carry out electronic structure calculations on their diiron cores as well
as on related synthetic compounds that closely resemble their biological counterparts. More generally, we seek to establish
a correlation between the electronic structure, the static and dynamic geometric
conformations, and the biological function of active sites in metalloproteins. |