What is AMS?
PDF Download - PRIME Lab Facilities (PDF)
DescriptionAccelerator mass spectrometry (AMS) is a technique for measuring long-lived radionuclides that occur naturally in our environment. AMS uses a particle accelerator in conjunction with ion sources, large magnets, and detectors to separate out interferences and count single atoms in the presence of 1x1016 (ten thousand million million) stable atoms. At PRIME Lab we measure six different cosmogenic radionuclides. They are used for a wide variety of dating and tracing applications in the geological and planetary sciences, archaeology, and biomedicine.
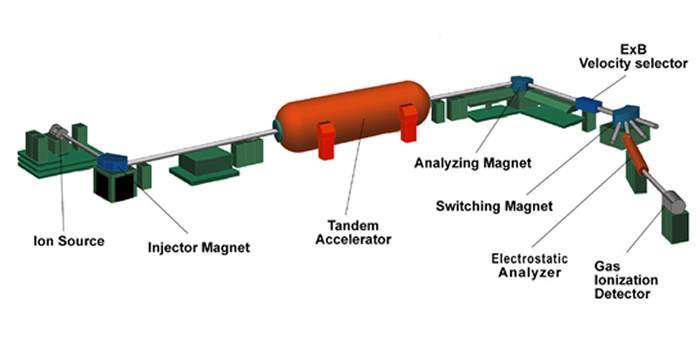
The following is a brief description of each element of the AMS system.
The ion source produces a beam of ions (atoms that carry an electrical charge) from a few milligrams of solid material. The desired element is first chemically extracted from the sample (for example, a rock, rain water, a meteorite) then it is loaded into a metal holder (Stainless Steel, Aluminum or Copper, depending on the desired element) and inserted into the ion source through an ultra-high vacuum sample changer. Atoms are sputtered from the sample by cesium ions which are produced on a hot spherical surface, called an ionizer and focused to a small spot on the sample. Negative ions produced on the surface of the sample are extracted (by electrostatic attraction) from the ion source and sent down the evacuated beam line towards the first magnet. At this point the beam is about 10 microamps which corresponds to 1013 ions per second (mostly the stable isotopes).
The injector magnet bends the negative ion beam by 90° to select the mass of interest, a radioisotope of the element inserted in the sample holder, and reject the much-more-intense neighboring stable isotopes. Several vacuum pumps remove all the air from the beamline so the beam particles have a free path. There are still lots of molecules and isobars (isotopes of neighboring elements having the same mass) that must be removed by more magnets after the accelerator.
The tandem accelerator consists of two accelerating gaps with a large positive voltage in the middle. Think of it as a bridge that spans the inside of a large pressure vessel containing CO2 and N2 insulating gas at a pressure of over 10 atmospheres. The bridge holds two long vacuum tubes with many glass (electrically insulating) sections. The center of the accelerator, called the terminal, is charged to a voltage of up to 10 million volts by two rotating chains. The negative ions traveling down the beam tube are attracted (accelerated) towards the positive terminal. At the terminal they pass through an electron stripper, either a gas or a very thin carbon foil, and emerge as positive ions. These are repelled from the positive terminal, accelerating again to ground potential at the far end. The name tandem accelerator comes from this dual acceleration concept. The final velocity is a few percent of the speed of light or about 50 million miles per hour.
The analyzing and switching magnets select the mass of the radionuclide of interest, further reducing the intensity of neighboring stable isotopes. In addition, they eliminate molecules completely by selecting only the highly charged ions that are produced in the terminal stripper. (Highly charged molecules are unstable since they are missing the electrons that bind the atoms together). Isotope ratios are measured by alternately selecting the stable and radioisotopes with the injector and analyzing magnets.
The electrostatic analyzer is a pair of metal plates at high voltage that deflects the beam to the left by 20 degrees. This selects particles based on their energy and thus removes the ions that happen to receive the wrong energy from the accelerator.
The gas ionization detector counts ions one at a time as they come down the beamline. The ions are slowed down and come to rest in propane gas. As they stop, electrons are knocked off the gas atoms. These electrons are collected on metal plates, amplified, and read into the computer. For each atom, the computer determines the rate of energy loss and from that deduces the nuclear charge (element atomic number) to distinguish interfering isobars.