 |
Prof. Francis RobicheauxI've been a
professor of physics at Purdue University since 2013 (previously at
Auburn University 1993-2013). My research area is Theoretical Atomic
Physics, focusing on coherence and decoherence in quantum
systems, many body processes when photons interact with many atoms,
highly
excited (Rydberg) atoms, strong fields, and
ultracold plasmas. My group typically consists of undergrads,
grad students, and postdocs. I'm a member of the ALPHA collaboration:
the first group to trap the antimatter version of the Hydrogen atom and
the only group to quantitatively measure its properties.
|
|
|
Perturbed Rydberg Atoms(wikipedia link to Rydberg atoms)For many years, we have had a strong effort in studying highly excited atoms and molecules. The interest in this area is that highly excited atoms can be understood using classical and quantum ideas. They also have been very common elements in quantum simulators and proposed quantum computers. We are particularly interested in the properties of these electronic states when they are strongly perturbed. The perturbations break the spherical symmetry of the atom and give rise to an interesting interplay between different types of motion. Also, specific and well controlled experiments can be performed on these systems. 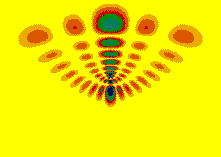 For these calculations, we often solve both the classical equations of motion and the time dependent Schrodinger equation which governs the quantum behavior. Depending on the system being studied, the quantum calculations solve for the wavefunction can be converged for distances out to 10-6 m from the ion and include angular momentum up to 1000 hbar. Below is a brief description of results in two recent publications. P. Giannakeas, M. T. Eiles, F. Robicheaux, and J. M. Rost, "Dressed ion-pair states of an ultralong-range Rydberg molecule," Phys. Rev. Lett. 125, 123401 (2020). PDF (2090 kB) This paper predicts the existence of a new class of states when a cold atom perturbs a Rydberg state. The resulting energy versus distance from the ionic core of the Rydberg state to the cold atom leads to quantized motion that looks like that of a hydrogen atom with tiny charges for the proton and electron. The resulting energy levels are those of hydrogen with a surprisingly small Rydberg constant. A.S. Stodolna, F. Lepine, T. Bergeman, F. Robicheaux, A. Gijsbertsen, J.H. Jungmann, C. Bordas, and M.J.J. Vrakking, "Visualizing the coupling between red and blue Stark states using photoionization microscopy," Phys. Rev. Lett. 113, 103002 (2014). PDF (1210 kB) This paper is a collaboration between experiment and theory to study coupling between different types of states of atoms in electric fields. Electric fields can mix the angular momentum of a weakly bound electron giving states with qualitatively different character. Some have very large dipole moments aligned with the electric field and some are anti-aligned. For some states, small changes in electric field strength can cause states with qualitatively different character to have similar energy. Since states with nearly the same energy can be mixed by small perturbations, the small region occupied by the core electrons can lead to nearly complete mixing between the states. We used similar techniques to study H atoms: A.S. Stodolna, A. Rouzee, F. Lepine, S. Cohen, F. Robicheaux, A. Gijsbertsen, J.H. Jungman, C. Bordas, and M.J.J. Vrakking, "Hydrogen atoms under magnification: direct observation of the nodal structure of Stark states," Phys. Rev. Lett. 110, 213001 (2013). PDF (1,150 kB) Physics Focus (viewpoint) and in S. Cohen, M.M. Harb, A. Ollagnier, F. Robicheaux, M.J.J. Vrakking, T. Barillot, F. Lepine, and C. Bordas, "Wave function microscopy of quasibound atomic states," Phys. Rev. Lett. 110, 183001 (2013). PDF (481 kB) Five Recent Publications
P. Giannakeas, M. T. Eiles, F. Robicheaux, and J. M. Rost, "Generalized local frame-transformation theory for ultralong-range Rydberg molecules," Phys. Rev. A 102, 033315 (2020). PDF (2000 kB) E. Moufarej, M. Vielle-Grosjean, G. Khalili, A.J. McCulloch, F. Robicheaux, Y.J. Picard, and D. Comparat, "Forced field ionization of Rydberg states for the production of monochromatic beams," Phys. Rev. A 95, 043409 (2017). PDF (1080 kB) X. Wang and F. Robicheaux, "Energy shift and state mixing of Rydberg atoms in ponderomotive optical traps," J. Phys. B 49, 164005 (2016). PDF (934 kB) P. Giannakeas, Chris H. Greene, and F. Robicheaux, "Generalized local-frame-transformation theory for excited species in external fields," Phys. Rev. A 94, 013419 (2016). PDF (486 kB) S. Cohen, M. M. Harb, A. Ollagnier, F. Robicheaux, M. J. J. Vrakking, T. Barillot, F. Lepine, and C. Bordas, "Photoionization microscopy of the lithium atom: Wave-function imaging of quasibound and continuum Stark states," Phys. Rev. A 94, 013414 (2016). PDF (1650 kB) |
robichf[at]purdue.edu Links: |
